FIRST ON THE DAILY SIGNAL—The emergency room visitation rate for abortion complications appears to have increased since the U.S. Food and Drug Administration removed guardrails on the use of the abortion pill.
A U.S. federal court on July 13, 2020, temporarily stopped the FDA from requiring that mifepristone, or the abortion pill, be dispensed in person. That injunction remained in place until it was temporarily reversed by the U.S. Supreme Court in January 2021.
In April 2021, the FDA again stopped requiring that abortion drugs be dispensed to women in person, which allowed women to receive them through telehealth appointments and by mail. The FDA has not enforced the in-person dispensing requirement ever since.
There’s now evidence that the FDA’s change in the restrictions for mifepristone since 2020 have increased the hospital emergency room visitation rate. That’s according to an analysis from Jonathan Abbamonte, senior research associate at the Center for Data Analysis at The Heritage Foundation, shared with The Daily Signal.
The Heritage analysis compares public access data on emergency room visits due to induced abortion complications in South Carolina, New Jersey, and Arkansas both before and after the FDA stopped enforcing the in-person dispensing requirement for the abortion pill.
The discovery comes shortly before a presidential election in which the Democratic nominee, Vice President Kamala Harris, has blamed what she called “Trump abortion bans” for killing women who die from abortion pill complications.
“Now we know that at least two women—and those are only the stories we know here in the state of Georgia—died because of a Trump abortion ban,” Harris claimed. “The reality is, for every story we hear of the suffering under Trump abortion bans, there are so many of the stories we’re not hearing, but where suffering is happening every day in our country.”
But until now, no studies to date had looked at the effect that expanding abortion access has had on maternal morbidity in the U.S.
Abbamonte’s study shows that perhaps the danger lies with the abortion pills, not abortion bans.
Looking at the raw data, the ER visitation rate due to abortion complications appears to have risen in South Carolina and Arkansas after the FDA’s policy change, while the trend in New Jersey is less clear.
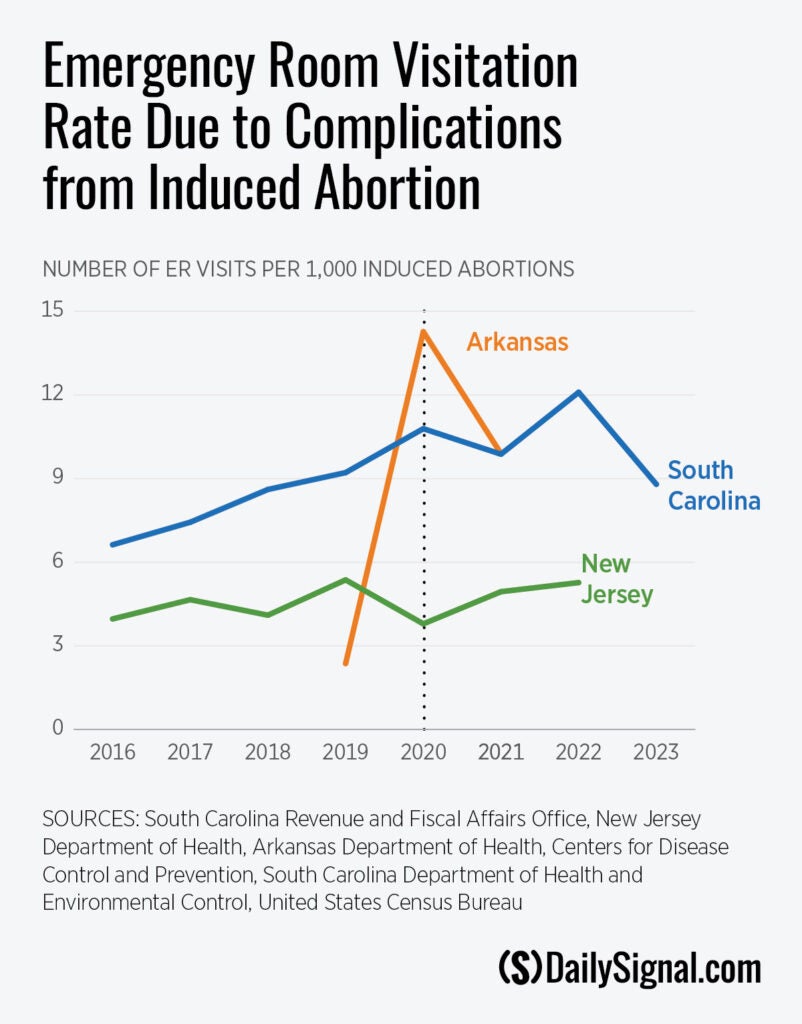
Arkansas had seven reported abortion complications in 2019, followed by 45 in 2020, and 31 in 2021. Arkansas banned abortion in 2022 with an exception to save the life of the mother.
The Abbamonte paper used a statistical model to estimate how much the ER visitation rate changed overall after the FDA stopped enforcing the in-person dispensing requirement. The model showed a statistically significant increase in the ER visitation rate after the FDA’s policy change.
Additionally, Abbamonte found significant evidence that the ER visitation rate for miscarriages increased following the introduction of the FDA’s new policy, after controlling for natural miscarriage-risk factors. That may indicate a rise in abortion complications being misreported as miscarriages, the report says.
The rate of ER visits reportedly due to miscarriage increased sharply in South Carolina and New Jersey after the FDA relaxed pill restrictions.
Women who go to the emergency room for abortion complications often do not disclose that they attempted to have a chemical abortion.
Dr. Cortney Draper, board-certified ER physician, said that, during her 10 years practicing in South Carolina, she has seen ER patients become increasingly hesitant to admit they took an abortion pill.
“I think they’re often told not to tell us,” Draper told The Daily Signal. “We’re kind of operating blindly in the emergency department because, all of a sudden, we find evidence that this baby is dead, or has already passed, or there’s partially retained products, all those kinds of things happen.”
Doctors may not be aware the complications they are seeing are from abortion drugs, as some women don’t disclose that information because they are embarrassed or because someone advised them not to, said Dr. Ingrid Skop, pro-life obstetrician-gynecologist and director of medical affairs for the Charlotte Lozier Institute.
“If a researcher is looking to determine how often or what percent of visits are related to abortion-drug complications,” Skop told The Daily Signal, “they’re only going to be seeing the tip of the iceberg if they’re only specifically looking for those that fall into the specific codes related to induced abortion.”
Skop said she expects the trend holds up more widely than in just South Carolina, New Jersey, and Arkansas.
“I would expect the complication rates to go up everywhere, because they are so increasingly promoting abortion drugs,” she said. “So, even if you’re a state that has no restrictions, and a woman walks into an abortion clinic, it is still the case that abortion drugs are going to be promoted to her over surgical abortion.”
Draper saw a patient in August who had retained fetal tissue from taking abortion pills in June. Because she was able to get the abortion pill without seeing a doctor due to the FDA’s lack of restrictions, she didn’t have a follow-up visit with a doctor after the chemical abortion and ended up in the emergency room with the early signs of an infection.
“It’s terrible health care,” she said.
Even in states where abortion is illegal with few or no exceptions, a woman would not be punished for taking an abortion pill regimen, so Draper is unsure why women are being advised to lie about it.
“We are fully treating these patients, whether they’ve taken an abortion regimen, whether they’re having a spontaneous miscarriage,” she said. “None of these laws restrict our ability to fully treat a patient, and the misinformation that’s being spread out there, claiming that we can’t is very concerning and harmful.”
“No woman is ever going to be not treated well because they already took an abortion pill regimen,” Draper continued. “We’re not going to allow them to just die in front of us, because we have an abortion law in our state. The abortion law only limits elective abortions, not emergency care.”
Complications from abortion pills can include sepsis, excessive bleeding, and pain, she said.
“A concern is if, if you have an ectopic pregnancy, and then we’re finding you’ve already taken an abortion pill regimen, that is not a safe way to treat it,” Draper said.
An ectopic pregnancy occurs when the unborn baby implants in the fallopian tubes instead of the uterus and thus cannot safely grow.
Skop once saw a woman in the emergency room in Texas who bled every day for two months after taking an abortion pill she got in California. She still had tissue in her uterus, so Skop performed a removal surgery.
“There are dozens of websites online where women can get access to these drugs,” Skop said. “Again, they come through the mail many times against state law, but there seems to be very little effort to try to enforce the law that prohibits mailing them, so they come into states with pro-life limitations. But also in Texas, a lot of women are driving. They’re driving to New Mexico. They’re driving to Colorado.”
The FDA should reinstate the mandatory in-person dispensing requirement for the abortion pill and reverse its decision allowing pharmacists to dispense the drug, according to Abbamonte’s report.
“We need better data about abortion,” Skop agreed. “If we’re saying that women need this [abortion pills], then we need to know what happens to women in the aftermath of abortion.”
Despite the limited data on abortion pill complications and ER visits, the stakes of the abortion pill being easily accessible without a mandatory doctor’s appointment are high for maternal morbidity, according to Abbamonte.
“Public health decisions must be evidence-based, and so far, the evidence points in the direction of a negative impact on maternal health and morbidity as a result of the FDA’s removal of the in-person dispensing requirement for the abortion pill,” Abbamonte wrote.
“The FDA should follow evidence-based policy on mifepristone and, thus, should immediately reinstate the mandatory in-person dispensing requirement for mifepristone and reverse its decision allowing retail pharmacies to dispense the drug,” he continued.
