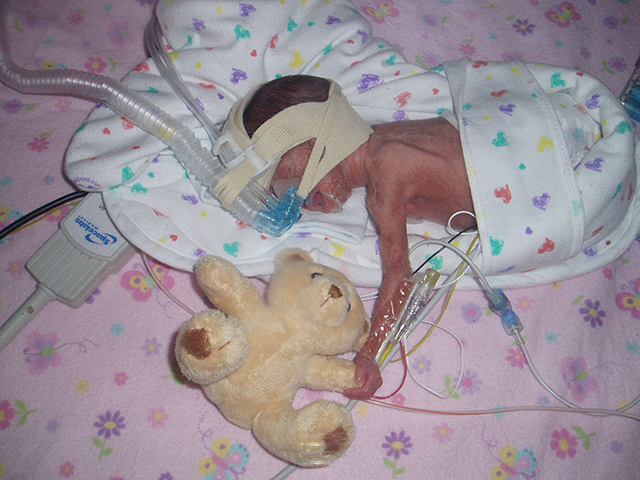
In May 2007, Carrie and Shawn Pratt agreed to sign up their severely premature daughter, Dagen, for a government-funded study being conducted at Duke University Hospital. The Pratts say they were told that researchers simply were gathering information to help other children.
“We never understood the study to be based on manipulating her oxygen level to meet [researchers’] needs,” Carrie Pratt says.
At issue is an experiment in which researchers at two dozen academic institutions randomly manipulated the oxygen levels of 1,316 extremely premature infants without providing their parents the full details of the methods and risks.
Confronted with the reality, which the Pratts discovered just last year, the West Virginia couple remain shell-shocked.
> > > Part 3 of 3: Re-examining how scientists seek consent in conducting tests on human subjects. Read Part 1: Did government’s experiment on preemies hide risks? Read Part 2: ‘Input’ stalls agency’s ethics probe in baby xygen trials
They already had lost a preemie son four years before Dagen was born. They say they can’t understand why medical professionals would have suggested enrolling their frail, newborn daughter in an experiment that could put her at further risk.
“When you have a small child, a micro-preemie, on a ventilator with see-through skin and fighting for her life … it is the most humbling, sad experience of your life,” Carrie Pratt says in an interview. “So, of course we would agree to participate in a study if it meant collecting info or data to help someone else. But certainly not at the expense of our daughter.”
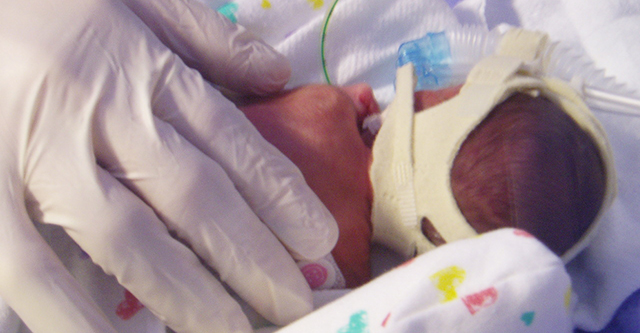
Tiny, fragile Dagen Pratt shortly after birth at Duke University Hospital. (Photo: courtesy Pratt family)
Today, the Pratts wonder whether the study, called SUPPORT, contributed to Dagen’s health issues. She suffered multiple incidences of collapsed lungs, breathing problems, and other life-threatening conditions. Diagnosed with retinopathy, Dagen had to have laser eye surgery when she was 2 months old. She has cerebral palsy. Now 7, she often wears orthotics on both legs.
“Do we blame SUPPORT?” Dagen’s mother asks.
The Academic Debate
The friendly sounding acronym SUPPORT stands for “Surfactant, Positive Airway Pressure, and Pulse Oximetry Randomized Trial.” The experiment, which cost taxpayers $20.8 million, was conducted at 23 academic institutions from 2005 to 2009 under the National Institutes of Health, part of the Department of Health and Human Services.
The consent form signed by parents did not disclose one controversial aspect of the experiment: The preemies’ oxygen monitors intentionally were altered to provide false readings so that medical staff wouldn’t be tempted to adjust the babies’ oxygen out of their study-assigned range.
More babies who received higher levels of oxygen ended up with serious vision disorders. The low-oxygen preemies were more likely to die. The results, published in the New England Journal of Medicine in May 2010, sparked ongoing ethical questions and complaints.
Little over nine months ago, hundreds of researchers and academics from around the globe gathered in person or via teleconference to address the supposed confusion surrounding informed consent in the wake of the SUPPORT controversy.
They came together Aug. 28 near the Capitol, in the Great Hall of the Hubert H. Humphrey Building, where HHS is headquartered. The meeting was the first step in the government’s effort to clarify and draft new guidance on the consent process for human research.
About half of the academics used the HHS forum to defend SUPPORT’s consent process. Some made the case that, in some instances, study subjects should be told less, not more.
If the process of informed consent becomes too off-putting, they argued, not enough patients would sign up for studies intended to advance what researchers consider the greater good.
Dr. John Lantos, director of pediatric bioethics at another study site, Children’s Mercy Hospitals and Clinics of Kansas City, Mo., said consent forms that make it sound like “death lurked at every corner” are counterproductive.
“[T]hey are not empowering people to make informed choices, they are scaring them into making uninformed ones,” Lantos said.
The discussion was academic until the Pratts took the stage—carrying pretty, 6-year-old Dagen, who was wearing a sundress and ponytails but looked fragile and thin in leg braces.

Carrie Pratt holds daughter Dagen, then 6, outside HHS headquarters last August. (Photo: Angela Bradbery/Public Citizen)
“We were guaranteed that the study wouldn’t hurt Dagen in any way, that it was just gathering information,” Shawn Pratt told the audience academics and research scientists, “and were shocked to learn the care she received was based not on what she needed, but on some protocol.”
Dagen’s father continued: “We want to know, as information comes in, why the risks and intent of the study were not clear. If it were clear, we wouldn’t have taken part in the study.”
‘That Wasn’t Clear?’
At least one of three HHS panelists who moderated the meeting appeared dumbfounded by the Pratts’ personal story after lofty discussions about the greater good.
The HHS ethics office director, Dr. Jerry Menikoff, was on the panel, but he wasn’t the one who spoke up.
Rather, it was Dr. Robert Temple, deputy director for clinical science at the Food and Drug Administration’s Center for Drug Evaluation and Research.
“Just to be sure I understood: You got some kind of consent form, but I take it you’re saying that you couldn’t tell from that, that there were actually two things that she was going to be randomized to?” Temple asked the Pratts. “But that wasn’t clear? Is that what you’re saying?”
“They said they are collecting data, that don’t worry, she is going to be cared for,” Carrie Pratt answered.
“So they didn’t really communicate that it was in fact an experiment?” Temple asked.
“No,” Dagen’s mom replied.
Sharrissa Cook also spoke about her son, Dreshan, at the HHS meeting. Cook was just 25 weeks into her pregnancy when she gave birth to a critically ill baby boy in October 2006. She is now part of a lawsuit alleging that the University of Alabama at Birmingham Hospital, the lead study site, misled her and other SUPPORT parents.
Dreshan, who weighed a fragile 1 pound, 11 ounces at birth, faces a myriad of health problems at age 7.
“Had I known the full extent of the study, I would not have given my consent. … I unknowingly placed my son in harm’s way,” Cook said. “I trusted them with my baby’s life … My son is a live, breathing human being. He is not simply a subject.”
HHS has yet to issue conclusions about what occurred during the multiyear experiment on preemies.
‘A Lot of Rationalization’
As Cook, the Pratts and other parents demand answers, a source who conducts clinical trials—but wasn’t involved in SUPPORT—provides insight into research dynamics.
Scientists and other researchers face “such incredible pressure today to advance research and their own position and standing in the research community,” the source says. As a result, “a lot of rationalization can take place” on the question of what to tell human test subjects.
Or in this case, what to tell their anxious and vulnerable parents.
Some researchers appear to be turning criticism of SUPPORT on its head. They argue that informed consent should be suspended altogether in such studies, which they contend merely evaluated an already-approved, widely used “standard of care” treatment.
As a mother, Carrie Pratt says she understands researchers’ concern that if consent forms tell potential study participants too much, they might be scared off.
“[But] seriously, can anyone blame them?” she asks. “One thing is for sure, anyone approaching us to participate in a study can just keep walking.”
Dr. Michael Carome, a former leader of the HHS ethics office who now directs health research for the consumer watchdog group Public Citizen, says talk of withholding more information from test subjects is dangerous.

Former HHS ethics official Michael Carome, now with Public Citizen, faults HHS for not enforcing standards in the baby oxygen trials. (Photo: Angela Bradbery/Public Citizen)
Public Citizen continues to press HHS to address “ethical lapses” in the NIH-financed study, and to allow the ethics office to complete investigative and enforcement actions without interference.
Carome says he considers it “highly likely” that many, if not most, parents would have refused to enroll their babies in the NIH-financed study had they been “appropriately informed about the nature of the research and its risks.”
But the answer, Carome argues, isn’t to hide the risks.
“Some experiments maybe just can’t be done,” he says.